- Our Company
- Cementing Chemicals
- Production Chemicals
- Drilling Fluid Additives
- Stimulation Chemicals
- Industrial Cleaning Products
- Services
- Sustainability
- Contact Us
- Safety Data Sheets
- Client Log In
Resources > Glossary >
Acid Emulsifiers
Emulsions
Emulsions are a dispersion of one immiscible liquid in another immiscible liquid. The liquid phase that is dispersed is known as the internal phase or the dispersed phase, while the other liquid phase is called the external phase or the continuous phase.
The stability of emulsions depends on:
- The size of a particle
- The difference in density of phases
- The viscosity of the continuous phase
- The viscosity of the finished emulsion
- Particle loads (Potential Z)
- The nature, efficiency and quantity of the emulsifier.
- Storage (high and low temperatures, agitation and vibration, dilution or evaporation during storage or use)

Energy is required to create an emulsion, and stabilizers must accumulate at the interface between the liquids to prevent the emulsion from breaking.
Additionally, emulsions can be stable by the effect of stabilizer agents. Energy is required to create an emulsion, and stabilizers must accumulate at the interface between the liquids to prevent the emulsion from breaking. The most important emulsion stabilizers are fine particles of clay or other materials, asphaltenes, and surfactants.
Surfactants can break an emulsion, acting on stabilizing materials, in such a way that they remove them from the interfacial film surrounding a drop of the emulsion.
According to Ostwald’s rule, if one of the phases is present in more than 74%, it will constitute the external (or continuous) phase of the emulsion because it cannot occupy more than 74% in volume of the internal phase with rigid and identical spheres. However, there are emulsions with internal phase content greater than 74%, such as emulsions gels.
Type of emulsions
Several parameters can be useful to classify emulsions. One of them is the size of the drop. According to this variable, emulsions can be classified as macroemulsions or nanoemulsions.
Emulsions or macroemulsions refer to classical emulsions where the drop size exceeds the micrometer, a value corresponding to the minimum drop size produced by mechanical agitation. Macroemulsions are in a range of 1-100 μm, which can eventually be extended up to a range of 0.1-500 μm.
Below a droplet size of 100 nm, there is talk of nanoemulsions. These are also biphasic systems, but emulsification protocols and unconventional methods, such as phase inversion, are used for their formation.
There are also so-called microemulsions, which refers to single-phase systems where the surfactant makes possible the coexistence, on an almost molecular scale, of the water and oil phases. Microemulsions have dynamic microdomains, not necessarily spherical, typically in the order of 10-50 nm. In contrast to macro or nanoemulsions, micro-emulsions are thermodynamically stable systems.
Another type of classification is based on the composition of the immiscible phase, as detailed in the following table.
| Name | Description |
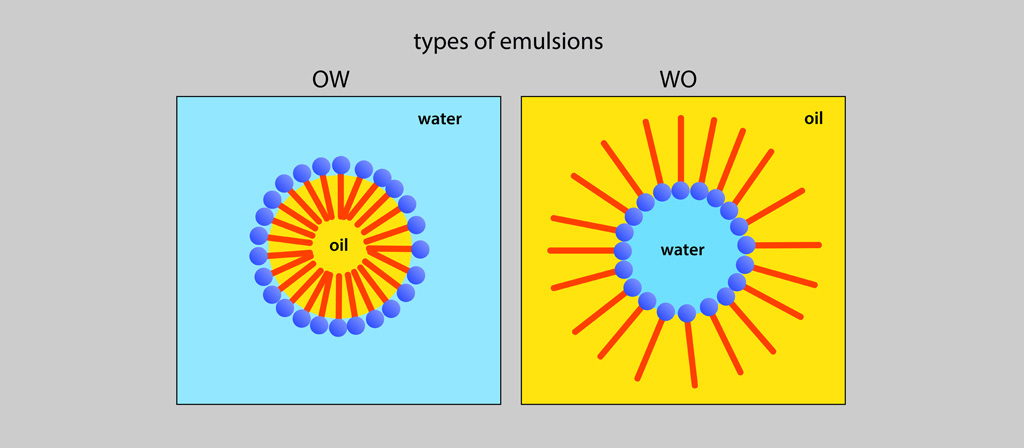
Water-in-Oil Emulsions (Simple direct emulsions) |
The drops are of water or an aqueous solution (dispersed or internal phase), and the phase in which the drops are immersed (continuous or external phase) is oil. |
Oil-in-Water Emulsions (Simple invert emulsions) O/W emulsions |
The drops are of oil (dispersed or internal phase), and the phase in which the drops are immersed (continuous or external phase) is aqueous. |
Multiple emulsions |
Multicompartitised systems, characterized by the coexistence of oil-in-water (O/W) and water-in-oil (W/O) emulsions, in which the dispersed phase globules contain within them equally small dispersed droplets. The most common are water-in-oil-in-water (W/O/W), although oil-in-water-oil (O/W/O) can also be used in specific applications. |
It can set up another type of classification according to the content of its internal phase:
- Between 0 and 5% internal phase, these are emulsions with low internal phase content, in which the drops do not present direct interactions with each other.
- Between 5 and 30% internal phase, are low internal phase content emulsions.
- Between 30 and 74% of internal phase, there are emulsions with medium internal phase content, whose properties have remarkable deviations to Newtonian behavior and depend considerably on its formulation and the emulsification protocol.
- More than 74% internal phase, these are emulsions with high internal phase content, in which the contact between the drops is very large, such as emulsions gels.
Characterization of emulsions
The most common properties for characterizing emulsions are:
- Zeta potential. It corresponds to the electrokinetic potential generated between the Stern layer of a charged particle and the diffuse layer of the medium in which it is found. This can be measured through electrophoretic mobility measures associated with dynamic light dispersion, which by mathematical equations convert these measures into zeta potential values using various models such as Smoluchowski. This technique is widely used to predict the kinetic stability of emulsions and suspensions stabilized by ionic surfactants.
- Determination of the droplet size distribution. In ordinary emulsions, we talk about the average diameter of the droplets. However, the only way to describe the geometry of an emulsion is by its distribution of droplet size.
- Microstructure analysis by optical microscopy. This property is measured by dynamic light scattering (DLS). The methods for measurement depending on the size of the particle: Rayleigh scattering, in which the size of the light beam is large, compared to the particle; o Mie scattering, in which the size of the particle is larger than the size of the light beam.
- Determination of physical stability by optical analysis of transmitted and backscattering.
- Rheological properties. Viscosity.
- Determination of oxidation propensity.
Characterization of emulsions
There are two main methods, both of disintegration, for preparing emulsions: direct and indirect. The indirect method consists of:
- Add the emulsifying agent to the oily phase (oil) and mix it until the surfactant is moisturized in the oily phase
- Add the oily phase to the aqueous with the neutralizing agent, if necessary
- Shake vigorously to cause an increase in viscosity and the formation of a creamy emulsion (reduce the size of the droplet)
The direct method is employed when the oily phase is partially polar, which can cause swelling (premature solvation) of the emulsifier, and consists of:
- Mix the emulsifier slowly with the water by stirring quickly
- Continue stirring and empty on the oily phase, neutralize
- Shake quickly to obtain a shiny product
In the case of gel-forming stabilizers around drops, it should avoid the use of homogenizing tanks as it can produce a variation in viscosity. These tanks produce large cutting forces so it can tear off the gel wrap, so the drops are ready to coalesce.
Emulsifiers
An emulsifier is an amphiphilic molecule of low or high molecular weight that tends to migrate and adsorb rapidly on the oil (apolar) and water (polar) interface, favoring the formation of drops with lower energy consumption. These compounds decrease interfacial tension and create a film at the interface.
Some of the desirable properties of an emulsifier agent are:
- Be surfactants to reduce surface tension below 10 dynes/cm.
- To be adsorbed quickly, around the dispersed drops, as a condensed, non-adhesive film that will prevent coalescence.
- To impart to droplets an electrical potential adequate to ensure mutual repulsion.
- Increase the viscosity of the emulsion.
- Be effective at a reasonably low concentration.
Type of emulsifiers
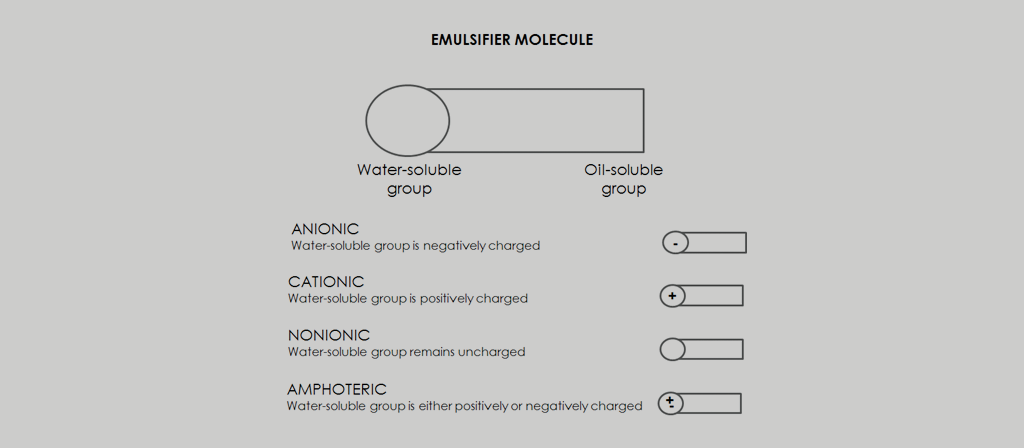
Emulsifiers can be classified into synthetic surfactants, natural active surface compounds and finely divided solids. One of the theories of emulsion formation is that the stability of an emulsion is due to the ionic charges of the internal phase. These charges can be obtained by ionization, droplet contact, or adsorption. Based on this theory, emulsifying agents can be represented as follows:
Among the natural compounds can be found rich in asphaltenes and resins, naphthenic acids, carboxylic acids, phenols, and other natural polymers such as gum arabic, gelatin, lecithin, and cholesterol.
Fine solids are emulsifiers that form particulate films around dispersed droplets and produce emulsions that, while coarse-grained, have considerable physical stability. It seems possible that any solid acted as an emulsifier of this kind, provided it is reduced to a fine enough powder. In practice, the most commonly used group of compounds is colloidal clays.
Use and action of synthetic emulsifiers
Surfactants for well-treatments are usually a combination of anionic and non-ionic surfactants. Anionic and cationic surfactants should not be used together, as the combination can produce an insoluble precipitate. Surfactants can be absorbed into solids to replace previously absorbed surfactants and provide solids with wettability characteristics of the strongest surfactant.
The action of surfactants can be divided according to the group load, as described in the table below.
| Emulsifier | Action |
|---|---|
Anionic |
|
Cationic |
|
Nionionic |
These surfactants are probably the most versatile of all well-stimulation surfactants, as these molecules do not ionize. In combination with other chemicals, non-ionic surfactants can provide other characteristics, such as high tolerance to hard water and acid pH. Most non-ionic surfactants are derivatives of ethylene oxide or mixtures of propylene-ethylene oxide. Since the water solubility of non-ionic s is due to the hydrogen bond or the oxygen attraction of water from ethylene oxide, this attraction is reduced to high temperatures and high concentrations of salt, causing most non-ionic surfactants to separate from the solution. |
Amphoteric |
These are molecules that contain acid and basic groups. At an acidic pH, the basic part of the molecule is ionized and provides a surface activity to the molecule. At a basic pH, the acidic part of the molecule is ''neutralized" and usually has less surface activity than at other pH values. There is limited use of amphoteric surfactants; however, some are being used as corrosion inhibitors. |
Acid Emulsifiers
Acidification is probably the most widely used conditioning and stimulation practice in the oil industry. By dissolving the acid-soluble components within the reservoir, it can be increased the productivity and injectability of the well.
In conventional acidification treatments different acids are used, the most common are: simple hydrochloric (HCl), acetic (CH3COOH), formic (HCOOH), gelled acids, VES acids, and emulsified acids.
Often, particularly in the presence of carbonates, it is desired to delay acidification so that the acid fluid can penetrate some distance from the well before reacting with the rock. One method of retardation is to emulsify the aqueous acid phase in kerosene in the form of W/O. For such emulsions are used petroleum sulfonates or carboxylic acid salts. With kerosene being the continuous phase, acid-rock contact does not occur immediately, and the emulsion can penetrate several meters from the well before all the acid is consumed.
Acid emulsifiers are formulations usually contain acid in the internal phase and 10 to 30% kerosene or diesel in the external phase. The increase in viscosity created by emulsification retards the rate of reaction of the acid with the rock, increasing the depth of penetration of the acid into the formation.
The acid-diesel emulsion has several advantages. In addition to its slow reaction rate with carbonated rocks, it has a relatively high viscosity. As a result, it has better scanning efficiency that will improve acid distribution in heterogeneous deposits. Also, the live acid does not come into contact with the pits' tubular; therefore, the level of corrosion is low. Also, the concentration of iron in the live acid that reaches the formation will be low and this reduces iron precipitation once is spent the acid.
Resources
Contact Us
Head Office
Al Moghera LLC
P.O.Box 4972
Abu Dhabi, United Arab Emirates
Tel. +971 2 5515454
Fax. +971 2 5515453
Plant
Plots 79 - 80 Sector MW-5 & Street 7
Mussafah Industrial Area
Abu Dhabi, United Arab Emirates
Tel. +971 2 5515454
Fax. +971 2 5515453

